Unveiling the Secrets of Molybdenum: Understanding Its Electron Configuration
Molybdenum is a fascinating element that has captured the imagination of scientists and engineers for centuries. Its unique properties have made it a valuable resource for everything from strengthening steel to producing catalysts. But what is it about molybdenum that makes it so special? The answer lies in its electron configuration. Understanding how the electrons are arranged in the atom can shed light on why molybdenum behaves the way it does in different chemical reactions and environments. In this article, we’ll delve into the secrets of molybdenum’s electron configuration and explore its implications for everything from industrial processes to biological systems.
What is Electron Configuration?
Before we dive into the specifics of molybdenum’s electron configuration, let’s take a moment to understand what we mean by that term. Electron configuration refers to the way in which electrons are arranged within an atom. Every atom contains a certain number of electrons, and these electrons occupy specific energy levels or orbitals. The electrons in the outermost energy level, known as the valence electrons, are the ones that are involved in chemical reactions.
The arrangement of these electrons can have a significant impact on the chemical properties of an element. For example, the number and arrangement of valence electrons can determine whether an element is a metal, nonmetal, or metalloid, and can affect its reactivity with other elements. By understanding the electron configuration of an element, scientists can gain insights into its chemical behavior and potential applications.
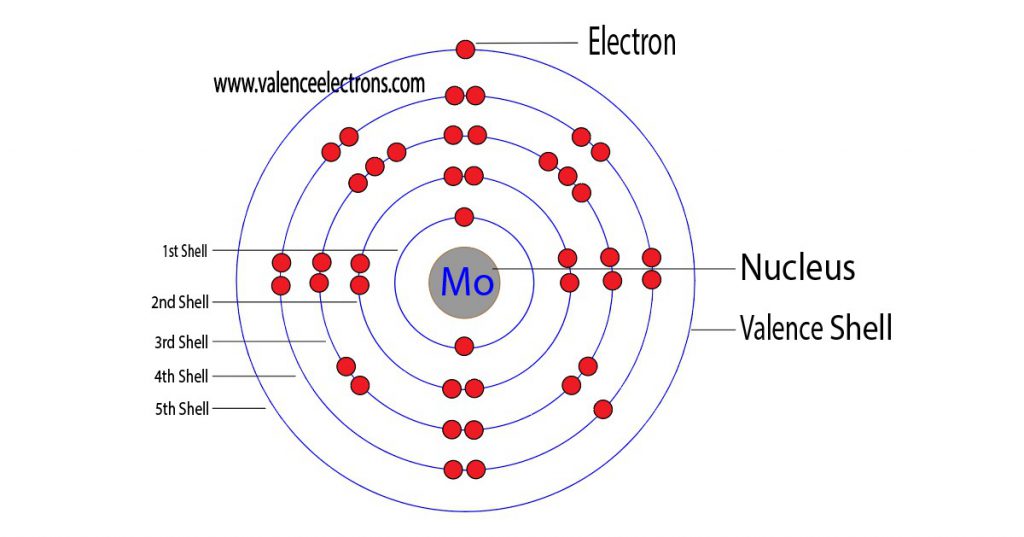
Understanding Molybdenum’s Electron Configuration
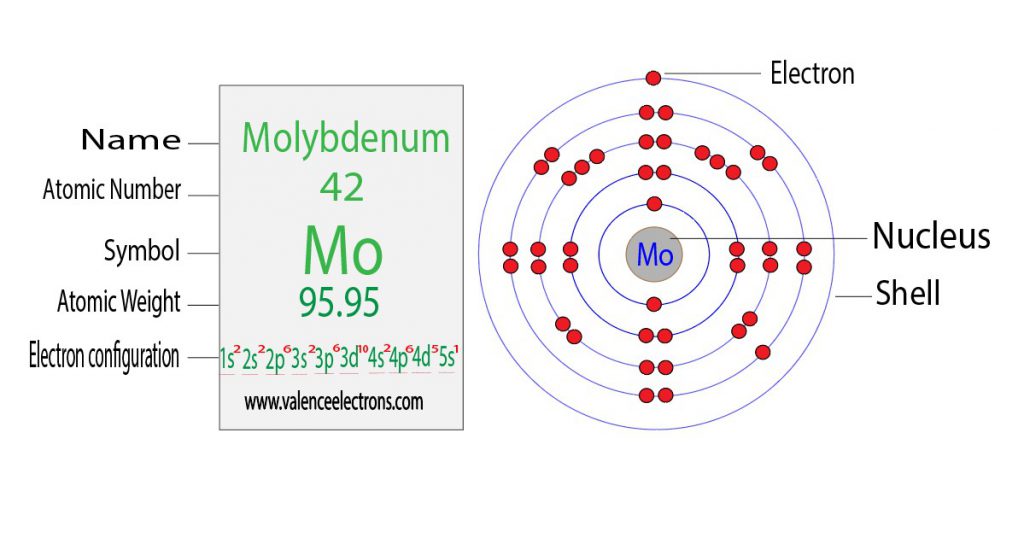
Now that we have a basic understanding of what electron configuration is, let’s turn our attention to molybdenum. Molybdenum is a transition metal that is located in the sixth row of the periodic table. It has an atomic number of 42, which means that it contains 42 protons and 42 electrons.
The electron configuration of molybdenum can be represented as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d5. This configuration indicates that molybdenum has five valence electrons, which are located in the 4d orbital. The presence of these valence electrons in the 4d orbital is what gives molybdenum its unique properties.
The Significance of Molybdenum’s Electron Configuration
So, what is it about molybdenum’s electron configuration that makes it so special? One important factor is the size of the 4d orbital. This orbital is relatively large compared to other orbitals, which makes it more likely that molybdenum will form chemical bonds with other elements.
Additionally, the position of the valence electrons in the 4d orbital means that they are shielded from the positive charge of the nucleus by the other electrons in the atom. This makes it easier for the valence electrons to interact with other atoms and molecules, which can lead to increased reactivity.
Another important aspect of molybdenum’s electron configuration is its ability to exist in multiple oxidation states. Molybdenum can form compounds in which it has a positive charge of +2, +3, +4, +5, or +6. This versatility is due in part to the presence of the valence electrons in the 4d orbital, which can be involved in a variety of chemical reactions.
Molybdenum’s Role in Biological Systems
Molybdenum’s unique properties make it an important element in biological systems. In particular, molybdenum is an essential component of several enzymes that are involved in processes such as nitrogen fixation and sulfur metabolism.
One example of a molybdenum-containing enzyme is nitrogenase, which is responsible for converting nitrogen gas into ammonia. This process is essential for the growth of plants and other organisms, as ammonia is a key component of amino acids and nucleotides.
Another molybdenum-containing enzyme is sulfite oxidase, which is involved in the metabolism of sulfur-containing amino acids. This enzyme plays a critical role in preventing the buildup of toxic sulfite compounds in the body.
Molybdenum in Industry and Technology
Molybdenum’s unique properties make it a valuable resource in a variety of industrial and technological applications. For example, molybdenum is often used as an alloying agent in steel production. When added to steel, molybdenum can increase its strength, toughness, and corrosion resistance.
Molybdenum is also used as a catalyst in a variety of chemical reactions. One example is the production of formaldehyde, which is used in the manufacture of plastics, textiles, and other materials. Molybdenum-based catalysts are also used in the production of sulfuric acid, a key component in many industrial processes.
In addition to its industrial uses, molybdenum is also used in electronics and other technologies. For example, molybdenum is used as a component in the electrodes of certain types of light bulbs and in the production of thin films for solar cells and other electronic devices.
Comparison with Other Transition Metals
While molybdenum is a unique element in many ways, it shares some similarities with other transition metals. For example, molybdenum, tungsten and chromium are also known for their high melting points and strength.
However, each of these elements has its own distinct electron configuration and properties. For example, tungsten has a similar electron configuration to molybdenum but has a higher melting point and is denser. Chromium, on the other hand, has a different electron configuration and is known for its corrosion resistance and ability to form colorful compounds.
Applications of Molybdenum
The unique properties of molybdenum make it a valuable resource in a variety of applications. Some of the key applications of molybdenum include:
- Steel production: Molybdenum is often used as an alloying agent in steel production to increase its strength and corrosion resistance.
- Catalysts: Molybdenum-based catalysts are used in a variety of chemical reactions, from the production of formaldehyde to the manufacturing of sulfuric acid.
- Electronics: Molybdenum is used in the production of thin films for solar cells and other electronic devices.
- Biological systems: Molybdenum is an essential component of several enzymes that are involved in processes such as nitrogen fixation and sulfur metabolism.
Conclusion: The Importance of Molybdenum’s Electron Configuration
In conclusion, molybdenum is a fascinating element that owes many of its unique properties to its electron configuration. The size and position of the valence electrons in the 4d orbital make molybdenum more likely to form chemical bonds and increase its reactivity. This, coupled with its ability to exist in multiple oxidation states, makes molybdenum a versatile element that has applications in everything from steel production to biological systems. By understanding the electron configuration of molybdenum and other elements, scientists can gain insights into their properties and potential applications, leading to new discoveries and innovations.
For more information about molybdenum metal and alloy materials, please visit https://www.samaterials.com/.



